Abstract
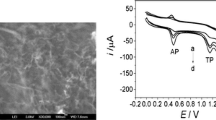
We have prepared a pyrolytic graphite electrode (PGE) whose surface is covered with a thin film of a nano-mixture of graphite/diamond (NGD). The electrode is shown to be capable of electrochemically sensing of tryptophan (TRP) and 5-hydroxytryptophan (HTRP). The presence of the NGD film resulted in a remarkable increase in the peak currents and sharpness of the waves so that submicromolar concentrations of TRP and HTRP become detectable. Potential scan rates, the pH of the solution, the accumulation conditions and the amount of the modifier were optimized via cyclic voltammetry. Linear sweep voltammetry, under optimized accumulation time and in open circuit operation, was applied to the determination of TRP and HTRP with detection limits (S/N = 3) of 30 nM (TRP) and 6 nM (HTRP). The electrode can be easily prepared, displays high sensitivity, sharp peaks, long-term stability, and remarkable voltammetric reproducibility and repeatability. These properties make the sensor suitable for the trace analysis of TRP and HTRP in pharmaceutical and clinical preparations.

A pyrolytic graphite electrode modified with a thin film of a nano-mixture of graphite/diamond. This electrochemical sensor applied for determination of tryptophan and 5-hydroxytryptophan in aqueous solutions. The modified electrode showed a remarkable increase in the peak currents and sharpness of the waves.




Similar content being viewed by others
References
Baron R, Sljukic B, Salter C, Crossley A, Compton RG (2007) Development of an electrochemical sensor nanoarray for hydrazine detection using a combinatorial approach. Electroanalysis 19:1062–1068
Campbell FW, Compton RG (2010) The use of nanoparticles in electroanalysis: an updated review. Anal Bioanal Chem 396:241–259
Shahrokhian S, Ghalkhani M (2010) Glassy carbon electrodes modified with a film of nanodiamond–graphite/chitosan: application to the highly sensitive electrochemical determination of Azathioprine. Electrochim Acta 55:3621–3627
Shenderova O, Jones C, Borjanovic D, Hens S, Cunningham G, Moseenkov S, Kuznetsov V, McGuire G (2008) Detonation nanodiamond and onion-like carbon: application in composites. Phys State Solidi 205:2245–2251
Zhao L, Takimoto T, Ito M, Kitagawa N, Kimura T, Kumatso N (2011) Choromatographic separation of highly soluble diamond nanoparticles prepared bypoylglycerol grafting. Angew Chem Int Ed 50:1388–1392
Yang G, Zheng W, Tian H, Wang X, Xu Q, Cheng J, Ho Y, Jiang Q (2008) Investigation on nanodiamond and carbon nanotube-diamond nanocomposite synthesized using RF-PECVD. Chem Vap Depos 14:236–240
Enoki T, Takai K, Osipov V, Baidakova M, Vul’ A (2009) Nanographene and nanodiamond: new members in the nanocarbon family. Chem Asian J 4:796–804
Orlanducci S, Tamburri E, Terranova ML, Rossi M (2008) Nanodiamond-coated carbon nanotubes: early stage of the CVD growth process. Chem Vap Depos 14:241–246
Krueger A (2008) New carbon materials: biological applications of functionalized nanodiamond materials. Chem Eur J 14:1382–1390
Kaplan RD, Mann JJ (1982) Altered platelet serotonin uptake kinetics in schizophrenia and depression. Life Sci 31:583–588
Nakajima T, Kudo Y, Kaneko Z (1978) Clinical evaluation of 5-hydroxy-L-tryptophan as an antidepressant drug. Folia Psychiatr Neurol Jpn 32:223–230
Martins AC, Gloria MB (2010) Changes on the levels of serotonin precursors – tryptophan and 5-hydroxytryptophan – during roasting of Arabica and Robusta coffee. Food Chem 118:529–533
Kema IP, de Vries EGE, Muskiet FAJ (2000) Clinical chemistry of serotonin and metabolites. J Chromatogr B 747:33–48
Neels OC, Jager PL, Koopmans KP, Eriks E, de Vries EGE, Kema IP, Elsinga PH (2006) Development of a reliable remote-controlled synthesis of b-[11C]-5-hydroxy-L-tryptophan on a Zymark robotic system. J Label Compd Radiopharm 49:889–895
Kochen W, Steinhart H (1994) L-tryptophan, current prospects in medicine and drug safety. de-Gruyter, Berlin
Wang W, Qiu B, Xu X, Zhang L, Chen G (2004) Separation and determination of L-tryptophan and its metabolites by capillary micellar electrokinetic chromatography with amperometric detection. Electrophoresis 25:903–910
Wu JM, Shi QZ, Wang GS, Peng TZ (1994) Potato-juice modified carbon paste electrode for the determination of tryptophan. Chin J Anal Chem 22:599–601
Wang GS, Peng TZ, Shen BZ, Zhu PL, Qu LN (1993) Determination of amino acid with montmorillonite modified carbon paste electrode I. Determination of tryptophan. Chin J Anal Chem 21:779–782
Huang KJ, Xu CX, Xie WZ, Wang W (2009) Electrochemical behavior and voltammetric determination of tryptophan based on 4-aminobenzoic acid polymer film modified glassy carbon electrode. Colloids Surf B: Biointerface 74:167–171
Shahrokhian S, Fotouhi L (2007) Carbon paste electrode incorporating multi-walled carbon nanotube/cobalt salophen for sensitive voltammetric determination of tryptophan. Sens Actuators B 123:942–949
Nana CG, Feng ZZ, Li WX, Ping DJ, Qin CH (2002) Electrochemical behavior of tryptophan and its derivatives at a glassy carbon electrode modified with hemin. Anal Chim Acta 452:245–254
Wu FH, Zhao GC, Wei XW, Yang ZS (2004) Electrocatalysis of tryptophan at multi-walled carbon nanotube modified electrode. Microchim Acta 144:243–247
Fan Y, Liu JH, Lu HT, Zhang Q (2011) Electrochemistry and voltammetric determination of L-tryptophan and L-tyrosine using a glassy carbon electrode modified with a Nafion/TiO2-graphene composite film. Microchim Acta 173:241–247
Banks CE, Compton RG (2006) New electrodes for old: from carbon nanotubes to edge plane pyrolytic graphite. Analyst 131:15–21
Wrona MZ, Dryhurst G (1990) Oxidation chemistry of 5- hydroxytryptamine. J Electroanal Chem 278:249–267
Zhang Z, Wang E (2000) Electrochemical principles and methods. Science, Beijing, p 242
Jin GP, Peng X, Chen QZ (2008) Preparation of novel arrays silver nonoparticles modified polyrutin coat-paraffin-impregnated graphite electrode for tyrosine and tryptophan’s oxidation. Electranalysis 20:907–915
Liu Y, Xu L (2007) Electrochemical sensor for tryptophan determination based on copper-cobalt hexacyanoferrate film modified graphite electrode. Sensors 7:2446–2457
Acknowledgements
The authors gratefully acknowledge the support of this work by the Research Council and the Center of Excellence for Nanostructures of the Sharif University of Technology, Tehran, Iran.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 475 kb)
Rights and permissions
About this article
Cite this article
Shahrokhian, S., Bayat, M. Pyrolytic graphite electrode modified with a thin film of a graphite/diamond nano-mixture for highly sensitive voltammetric determination of tryptophan and 5-hydroxytryptophan. Microchim Acta 174, 361–366 (2011). https://doi.org/10.1007/s00604-011-0631-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-011-0631-2